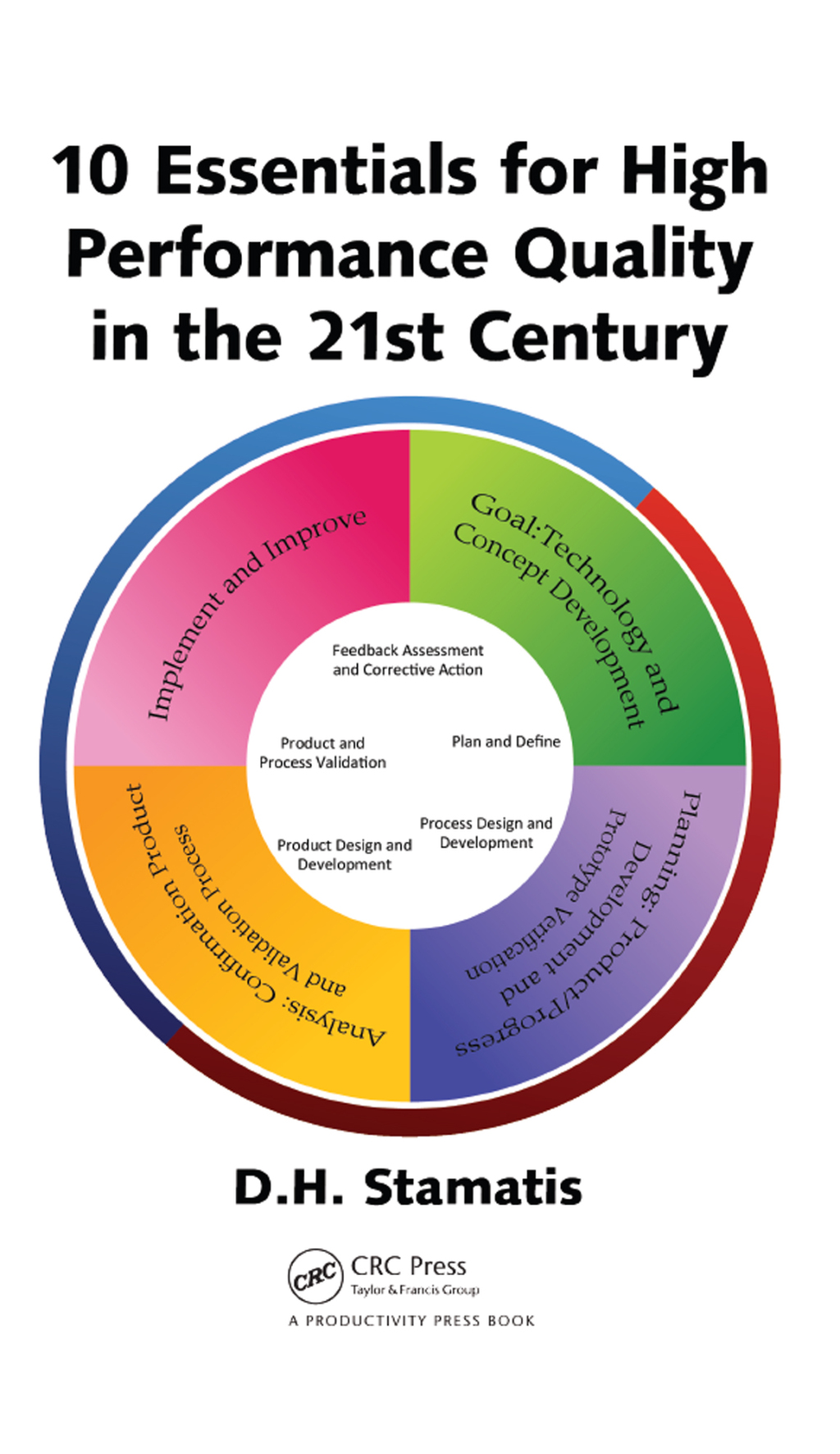
Description
Good Manufacturing Practice (GMP) ensures medicinal products are produced consistently and controlled to the quality standards appropriate for their intended use and as required by product specifications or marketing authorization. Annex 11 details the European Medicines Agency (EMA) GMP requirements for computer systems. The purpose of Annex 11 is to providethe EMA healthcare industry with consistent criteria for effective implementation, control, and use of computer systems. EU Annex 11 Guide to Computer Validation Compliance for the Worldwide Health Agency GMP supplies practical information to facilitate compliance with computer system GMP requirements, while highlighting and integrating the Annex 11 guidelines into the computer compliance program. The ideas presented in this book are based on the author’s 25 years of experience with computer validation in the healthcare industry with various computer systems development, maintenance, and quality functions. The book details a practical approach to increase efficiency and to ensure that software development and maintenanceare achieved correctly. Examining the implementation of the computer systems validation entirely based on EU Annex 11, the book includes examples from laboratory, clinical, and manufacturing computer systems. It also discusses electronic record integrity associated with stored information.